
Study completion will be end of April 2014. Group, 70 patients will be recruited from October 2013 to end of January 2014 and study The investigators will use FDA approved portable Ultraviolet B lamp for the intervention Vitamin D efficiently and thus have a high prevalence of vitamin D deficiency, leading to low These patients are not able to absorb oral

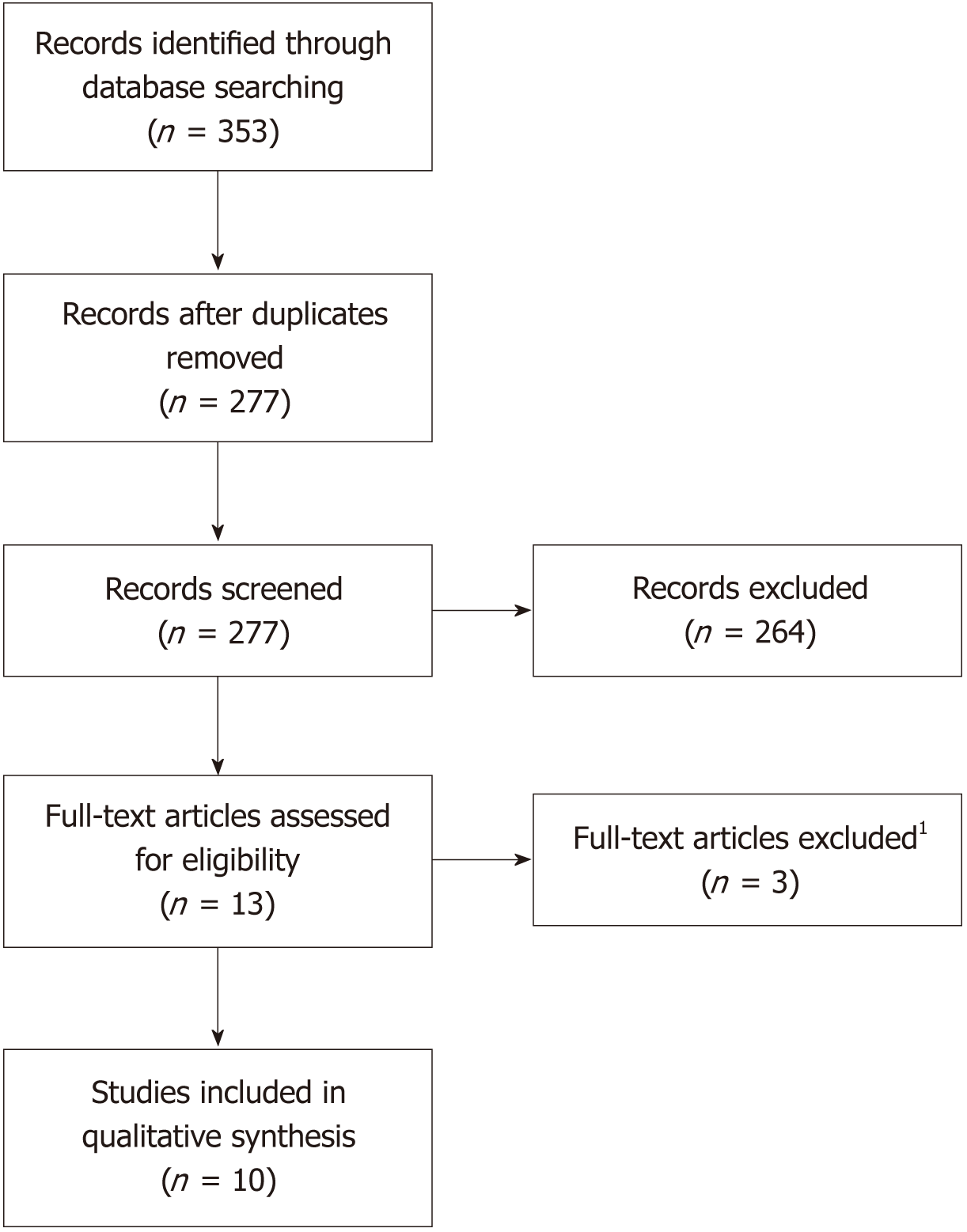
NCT ID NCT01930539 Status Completed Phase N/A Start date October 2013 Completion date December 2015 First recieived date Official title Treatment of Vitamin D Deficiency in Intestinal Rehabilitation Clinic Patients With a Portable Ultraviolet B Lamp Brief summary This is a randomized, controlled, unblinded pilot study for patients with vitamin Dĭeficiency in Intestinal Rehabilitation clinic.


 0 kommentar(er)
0 kommentar(er)
